Phenylketonuria (PKU)
Cure Program
About PKU
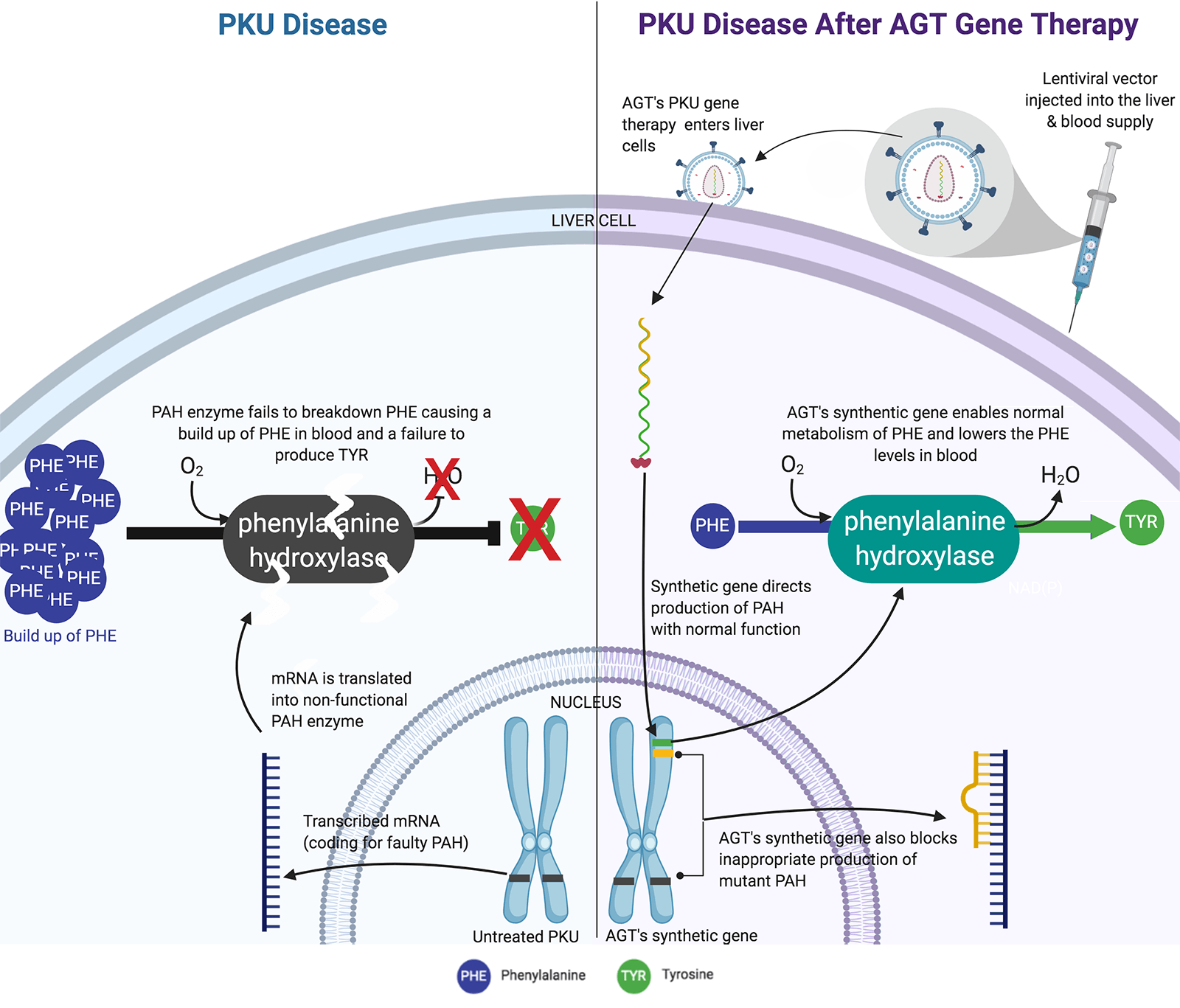
Inherited monogenic diseases generally involve loss of function due to gene mutations. Phenylketonuria (PKU) is one of the most common monogenic rare diseases with annual incidence in the United States of approximately 1 case among 13,500 live births. PKU is caused by loss of function mutations in the gene Pah that encodes the enzyme phenylalanine hydroxylase (PAH). The loss of PAH function leads to excess accumulation of the amino acid phenylalanine (Phe), which reaches toxic levels in blood without strict dietary control. AGT™ is targeting PKU with a therapeutic strategy based on proprietary lentivirus vectors for modifying human liver to restore normal PAH activity and reduce Phe levels. The general nature and efficiency of lentivirus vectors plus unique features of our proprietary modifications are particularly suitable for permanent correction of PKU after a single therapeutic dose. The estimated North American and European markets for PKU treatments including medical food and supplements, exceeding $1 billion annually, could be replaced with our lentivirus vector gene therapy.
PKU is an autosomal recessive disorder due to bi-allelic mutation of the Pah gene meaning the affected person received a mutant gene from each parent. The normal PAH enzyme hydroxylates phenylalanine and converts it to tyrosine, which is the rate-limiting step in controlling phenylalanine levels. With insufficient PAH function, persons with PKU are incapable of metabolizing dietary phenylalanine (Phe), which accumulates to toxic levels, and consequently fails to generate sufficient tyrosine that is required for biosynthesis of key metabolites including dopamine. PKU disease may be managed by strict compliance to a medical food diet lacking Phe. Children born with PKU must immediately and exclusively adhere to this medical food diet or risk severe defects in normal development affecting the brain and major organs. Adults with PKU who do not maintain strict dietary control, and most do not for a variety of reasons, suffer neuropsychiatric impairments including poor behavioral restraint and limited executive function with a high risk of self-injury. There is an important need to develop therapies for PKU that will correct behavioral abnormalities in adults to improve their quality of life. Successful gene therapy for PKU in adults will enable treatment for infants to prevent early damage due to Phe accumulation and provide a life-long cure.
Choosing Lentivirus Vectors for PKU Therapy
Modern gene therapy relies on viral vectors for delivering genetic medicines to sites of disease. The field of viral vectors includes a surprising range of alternate approaches, but current clinical applications mainly use lentivirus (LV) or adeno-associated virus (AAV) vectors. These two platforms are distinct in many ways and vector choice will impact all stages of clinical investigation, product licensure and uptake by the PKU patient population.
The distinct advantages of lentivirus vectors for PKU therapy include:
AGT believes that LV gene therapy is the best choice for treating adults with classical PKU due to safety, durability and cost-effectiveness. Further, LV are the clear choice for PKU gene therapy in infants where the main concerns are safety and achieving life-long phenotypic correction.
AGT PKU Cure Project
We are committed to developing potent genetic therapies that are safe, efficacious, and cost-effective for the greatest number of individuals with disease. Our therapeutic strategy for PKU was guided by these principles and has resulted in clinical approaches based on new viral vector drugs that will enable children and adults with PKU to lead normal lives.
Lentivirus vectors for PKU therapy permanently insert a functional PAH gene in the person's DNA. The permanence of lentivirus vector therapy is especially important for treating neonates or young children. Our goal is to modify liver cells with lentivirus vectors and reconstitute the capacity for phenylalanine metabolism. In newborns or very young children the liver is approximately 1/7 the size it will be in adults. Were we to use a non-integrating viral vector, such as adeno-associated virus (AAV), the initial dose given to a child would be diluted more than seven-fold during life and would quickly fall below levels needed to control disease. Integrating lentivirus vectors are ideally suited for permanent genetic medicines because they deliver an integrated transgene that replicates with host DNA during every cell division and the genetic medicine dose increases in proportion to growth of the liver. We are committed to single-dose gene therapies that will provide permanent correction of the underlying defect in a safe and cost-effective manner. Our lentivirus vector program in PKU is designed specifically for these goals and is progressing rapidly towards clinical development.
How AGT's PKU Gene Therapy Works
PKU Advocacy
Advocacy and financial support are very important for sustaining research and development on new treatments for PKU. Advocates will play critical roles in recruitment for clinical trials and may contribute to understanding the risk benefit analysis for genetic medicines. Several advocacy groups already exist for PKU and AGT is committed to working with this community to advance toward goals of curing PKU and relieving suffering for many patients and their families.
Jerry Vockley, M.D., Ph.D.
University of Pittsburgh: Cleveland Family Endowed Chair in Pediatric Research |
Professor of Human Genetics
Children's Hospital of Pittsburgh: Chief of Medical Genetics | Director of the Center for Rare Disease Therapy


